 As anyone who’s been through withdrawal can attest, recovering from opioid addiction is possible, but not necessarily easy. With relapse rates between 40 and 60 percent, it’s clear we don’t have all the answers for the best way to quit an addiction. But, we could be getting closer.
As anyone who’s been through withdrawal can attest, recovering from opioid addiction is possible, but not necessarily easy. With relapse rates between 40 and 60 percent, it’s clear we don’t have all the answers for the best way to quit an addiction. But, we could be getting closer.
One possible answer is the NSS-2 Bridge, a unique medical device system developed by Innovative Health Solutions, designed to dramatically reduce opioid withdrawal symptoms. Resembling a hearing aid and worn behind the ear, the Bridge is being used by VA hospitals and addiction treatment centers like Continuum Recovery Center in Phoenix, AZ to help support patients in the very earliest stages of recovery from opioid withdrawal — a necessary first step to long-term recovery.
To understand how the Bridge device supports the opioid recovery process, we must first acknowledge the gaps in traditional opioid withdrawal.
Barriers to Conventional Opioid Withdrawal
One of the greatest barriers to opioid rehabilitation is the acute pain associated with discontinuation. The body and brain react to not having opioids in the system, creating intense levels of discomfort, craving, agitation and irritability. In a nutshell, these symptoms make the cycle of opioid addiction fiendishly difficult to break.
For a treatment center like Continuum Recovery Center, the goal of opioid withdrawal is to remove opioids from the body, manage symptoms, and transition the patient to medication-assisted treatment, or MAT. The problem is that patients generally must endure substantial pain for a time without medication. Because this discomfort is often debilitating, many patients are unsuccessful in completing the process. Some relapse right away.
The Bridge Supports Opioid Rehabilitation
The Bridge device sends gentle electrical impulses to specific branches of the cranial nerves and occipital nerves, quieting pain signals and relieving the symptoms of opioid withdrawal in as little as 10 minutes. The Bridge is non-invasive and medication-free, making it a great choice for men and women of all ages.
Placing the Bridge is simple
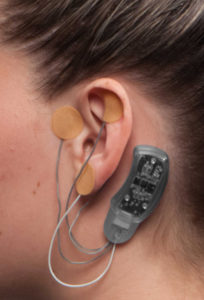 A qualified medical provider inserts small 1.5mm needles into the skin. The needles are attached to a wire, which is responsible for sending electrical pulses to the brain. The wires are then connected to the small plastic device.
A qualified medical provider inserts small 1.5mm needles into the skin. The needles are attached to a wire, which is responsible for sending electrical pulses to the brain. The wires are then connected to the small plastic device.
“The Bridge is literally a game-changer for patients struggling with the pain of opioid withdrawal,” said Executive Medical Director at Continuum Recovery Center, Walter Simmons, MD, MPH, FACEP. “In a quick visit to our office, I easily apply the device, which is completely unobtrusive and painless to wear. This technology is extremely effective at helping clients manage the symptoms of getting off opioids and moving into long-term recovery. Quite simply, opioid addicts fear to get clean because they know they’re going to get sick. The Bridge makes the process much more tolerable. ”
The Bridge is worn for up to five days, granting relief during the most difficult hours of opioid withdrawal. After this time, the device can be safely removed. While most of the physical symptoms should be over, it can take months or longer for the psychological symptoms of opioid addiction to subside. So ongoing behavioral therapy, counseling, support groups, and medication management aftercare are critical for ensuring long-term patient success.
Bridge Significantly Relieves Withdrawal Symptoms
The Bridge interrupts pain signals and reduces acute pain and discomfort. But, it doesn’t stop there. It makes the whole withdrawal process easier by relieving a number of withdrawal symptoms.
Opioid withdrawal effects are generally believed to be unpleasant but not fatal. This is not true. Death can occur, especially when the complications of withdrawal are mismanaged. For example, persistent vomiting and diarrhea can lead to dehydration, elevated blood sodium levels, and heart failure. The Bridge may not just decrease withdrawal symptoms but save lives.
The Bridge works best for:
- Anxiety
- Nausea
- Vomiting
- Insomnia
- Sweating
- Chills
- Agitation
- Teary eyes/runny nose
- Restlessness and tremors
A Single but Promising Case Study
The FDA was able to approve the Bridge device thanks to a case study conducted on patients during opioid detox. In the study, clinicians measured the device’s effectiveness using the Clinical Opiate Withdrawal Scale (COWS).
The breakdown of the scores are as follows:
5-12 = mild withdrawal symptoms
13-24 = moderate withdrawal symptoms
25-36 = moderately severe withdrawal symptoms
36+ = severe withdrawal symptoms
When using the Bridge device, patients reported a significant decrease in withdrawal symptoms such as joint aches, bone pain, sweating, anxiety, body shakes, pupil size and resting heart rate. Within 20 minutes of placement, patients reported their pain went from a 20 to a 5. Ninety percent of patients were able to move onto treatment.
To date, the Bridge has been tested on more than 30,000 patients and is used at a number of addiction treatment centers and VA hospitals across the United States.
Benefits of Using the Bridge Device
When a patient completes opioid withdrawal, they’re able to move onto medication-assisted treatment with drugs like methadone, buprenorphine or naltrexone. However, these drugs cannot be used during the detox process because patients cannot have any opioids in their systems. This is what makes an opioid withdrawal so debilitating and intolerable.
The Bridge acts as a “bridge” to MAT, hence its name. Here are the key benefits we do know about it:
Safe for most individuals. Men and women of any age can safely use the Bridge. This is important to know because addiction does not discriminate. Some adults who have never had addiction problems now struggle after being prescribed opioids.
Minimally invasive. It takes about 15 minutes to place the Bridge. This must be done by a qualified provider, as the device needs to match up to specific branches in the brain. After five days, the Bridge can be safely removed.
Low risk. When the device is attached, patients report feeling gentle pulses. They are subtle and easy to tolerate. No adverse reactions have been reported. Because the Bridge is non-medicinal, it can also be used in conjunction with other therapies.
FDA-approved. The Bridge was approved by the FDA in 2017 after reviewing data from the clinical study with 73 patients. It is available by prescription only.
Best Candidates for the Bridge
The Bridge is safe for almost all men and women struggling with opioid addiction and can offer patients comfort and reassurance as they enter rehabilitation. However, the manufacturer does not recommend the Bridge for people who are pregnant, have a history of seizures or have cardiac pacemakers.
Final Thoughts
Opioids are killing people at a considerable rate. For the first time, people are more likely to die from an opioid overdose than a car accident. Even for those who make it through withdrawal, only a small fraction of people make it to medical treatment.
The Bridge could be a solution that helps more people make it through the most painful days the process.
Currently, only select addiction treatment centers offer the Bridge. Continuum Recovery Center in Arizona is one of them.
To learn more about the Bridge device and how it’s being used to treat our own patients, contact us today. https://www.continuumrecoverycenter.com or call us. Your recovery starts with a simple phone call 855-732-3208.



































